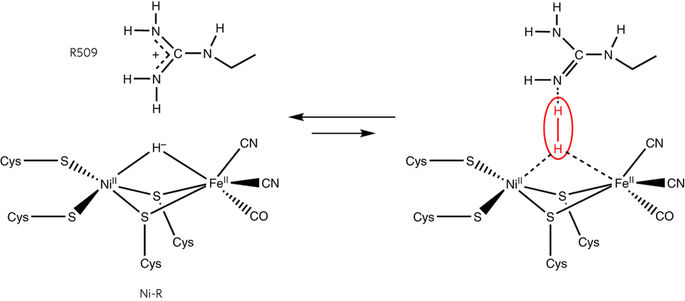
Exploiting Electrode Nanoconfinement to Investigate the Catalytic Properties of Isocitrate Dehydrogenase (IDH1) and a Cancer-Associated Variant
Ryan A. Herold, Raphael Reinbold, Clare F. Megarity, Martine I. Abboud, Christopher J. Schofield, Fraser A. Armstrong
ACS The Journal of Physical Chemistry Letters
[Link]
Exploiting Bidirectional Electrocatalysis by a Nanoconfined Enzyme Cascade to Drive and Control Enantioselective Reactions
Lei Wan, Rachel S. Heath, Clare F. Megarity, Adam J. Sills, Ryan A. Herold, Nicholas J. Turner, Fraser A. Armstrong
ACS Catalysis
[Link]
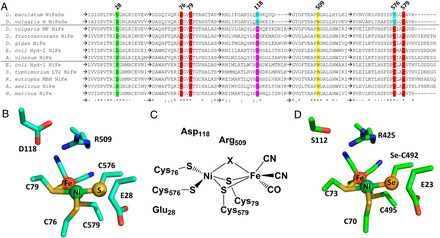
Selective cysteine-to-selenocysteine changes in a [NiFe]-hydrogenase confirm a special position for catalysis and oxygen tolerance
Rhiannon M. Evans, Natalie Krahn, Bonnie J. Murphy, Harrison Lee, Fraser A. Armstrong, Dieter Söll
PNAS
[Link]
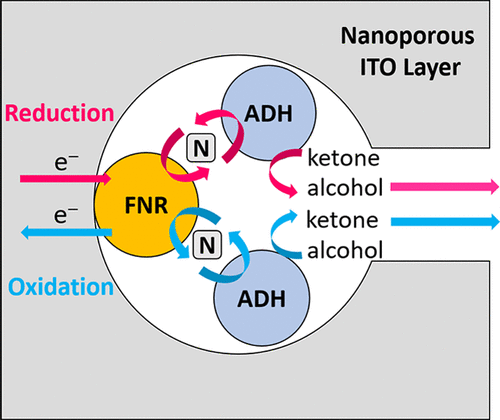
The power of electrified nanoconfinement for energising, controlling and observing long enzyme cascades
Giorgio Morello, Clare F. Megarity, Fraser A. Armstrong
Nature Communications
[Link]
Electron flow between the worlds of Marcus and Warburg
Clare F. Megarity, Bhavin Siritanaratkula, Ryan A. Herold, Giorgio Morello, Fraser A. Armstrong
J. Chem. Phys.
[Link]
Cascade biocatalysis in electrode nanopores
Clare F. Megarity, Fraser A. Armstrong
in Roadmap: Nanotechnology for catalysis and solar energy conversion
Banin et al
Nanotechnology
[Link]
Progress in Scaling up and Streamlining a Nanoconfined, Enzyme‐Catalyzed Electrochemical Nicotinamide Recycling System for Biocatalytic Synthesis
Beichen Cheng, Lei Wan, Fraser A. Armstrong
ChemElectroChem
[Link]
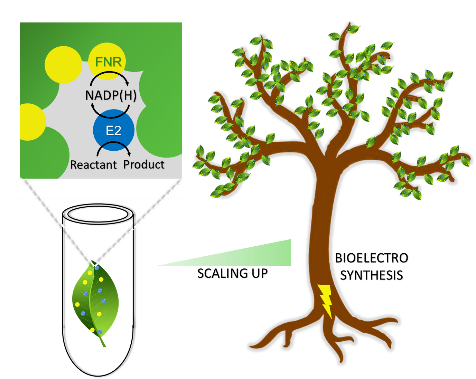
Aerobic Photocatalytic H2 Production by a [NiFe] Hydrogenase Engineered to Place a Silver Nanocluster in the Electron Relay
Liyun Zhang, Giorgio Morello, Stephen B. Carr, Fraser A. Armstrong
J. Am. Chem. Soc
[Link]
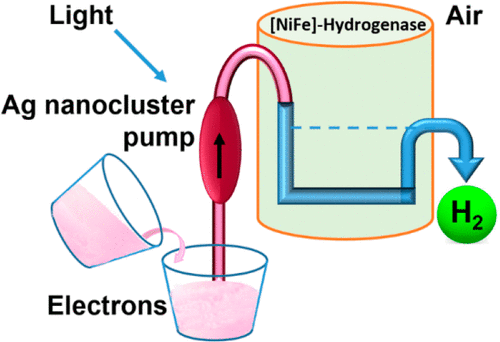
Efficient Electrocatalytic CO2 Fixation by Nanoconfined Enzymes via a C3-to-C4 Reaction That Is Favored over H2 Production
Giorgio Morello, Bhavin Siritanaratkul, Clare F. Megarity, Fraser A. Armstrong
ACS Catalysis
[Link]
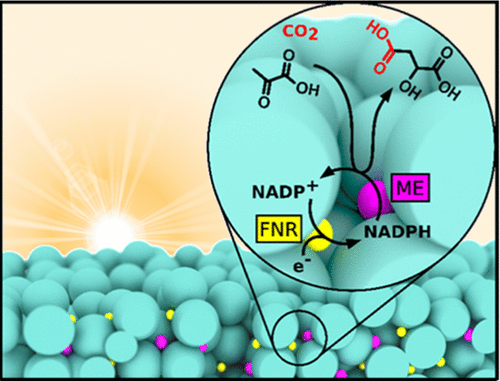
Electrified Nanoconfined Biocatalysis with Rapid Cofactor Recycling
Clare F. Megarity, Bhavin Siritanaratkul, Beichen Cheng, Giorgio Morello, Lei Wan, Adam J. Sills, Rachel S. Heath, Nicholas J. Turner, Fraser A. Armstrong
ChemCatChem
[Link]
Enzyme-catalysed enantioselective oxidation of alcohols by air exploiting fast electrochemical nicotinamide cycling in electrode nanopores
Lei Wan, Rachel S. Heath, Bhavin Siritanaratkul, Clare F. Megarity, Adam J. Sills, Matthew P. Thompson, Nicholas J. Turner, Fraser A. Armstrong
Green Chemistry
[Link]

Electrocatalytic Volleyball: Rapid Nanoconfined Nicotinamide Cycling for Organic Synthesis in Electrode Pores
Clare F. Megarity, Bhavin Siritanaratkul, Rachel S. Heath, Lei Wan, Giorgio Morello, Sarah R. FitzPatrick, Rosalind L. Booth, Adam J. Sills, Alexander W. Robertson, Jamie H. Warner, Nicholas J. Turner, Fraser A. Armstrong
Angewandte Chemie
[Link]
The value of enzymes in solar fuels research – efficient electrocatalysts through evolution
Rhiannon M. Evans, Bhavin Siritanaratkul, Clare F. Megarity, Kavita Pandey, Thomas F. Esterle, Selina Badiani and Fraser A. Armstrong
Chem. Soc. Rev.
[Link]
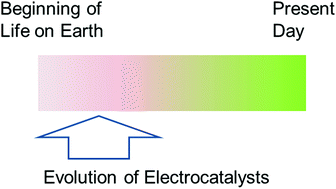
X-ray structural, functional and computational studies of the O2-sensitive E. coli hydrogenase-1 C19G variant reveal an unusual [4Fe–4S] cluster
A. Volbeda, J. M. Mouesca, C. Darnault, M. M. Roessler, A. Parkin, F. A. Armstrong and J. C. Fontecilla-Camps
Chem. Commun.
[Link]
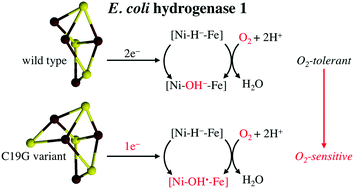
Mechanistic Exploitation of a Self-Repairing, Blocked Proton Transfer Pathway in an O-2-Tolerant [NiFe]-Hydrogenase
Rhiannon M. Evans, Philip A. Ash, Stephen E. Beaton, Emily J. Brooke, Kylie A. Vincent, Stephen B. Carr and Fraser A. Armstrong
J. Am. Chem. Soc
[Link]
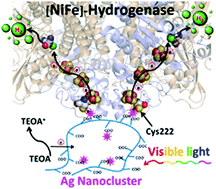
Direct visible light activation of a surface cysteine-engineered [NiFe]-hydrogenase by silver nanoclusters
Liyun Zhang, Stephen E. Beaton, Stephen B. Carr and Fraser A. Armstrong
Energy Environ. Sci.
[Link]
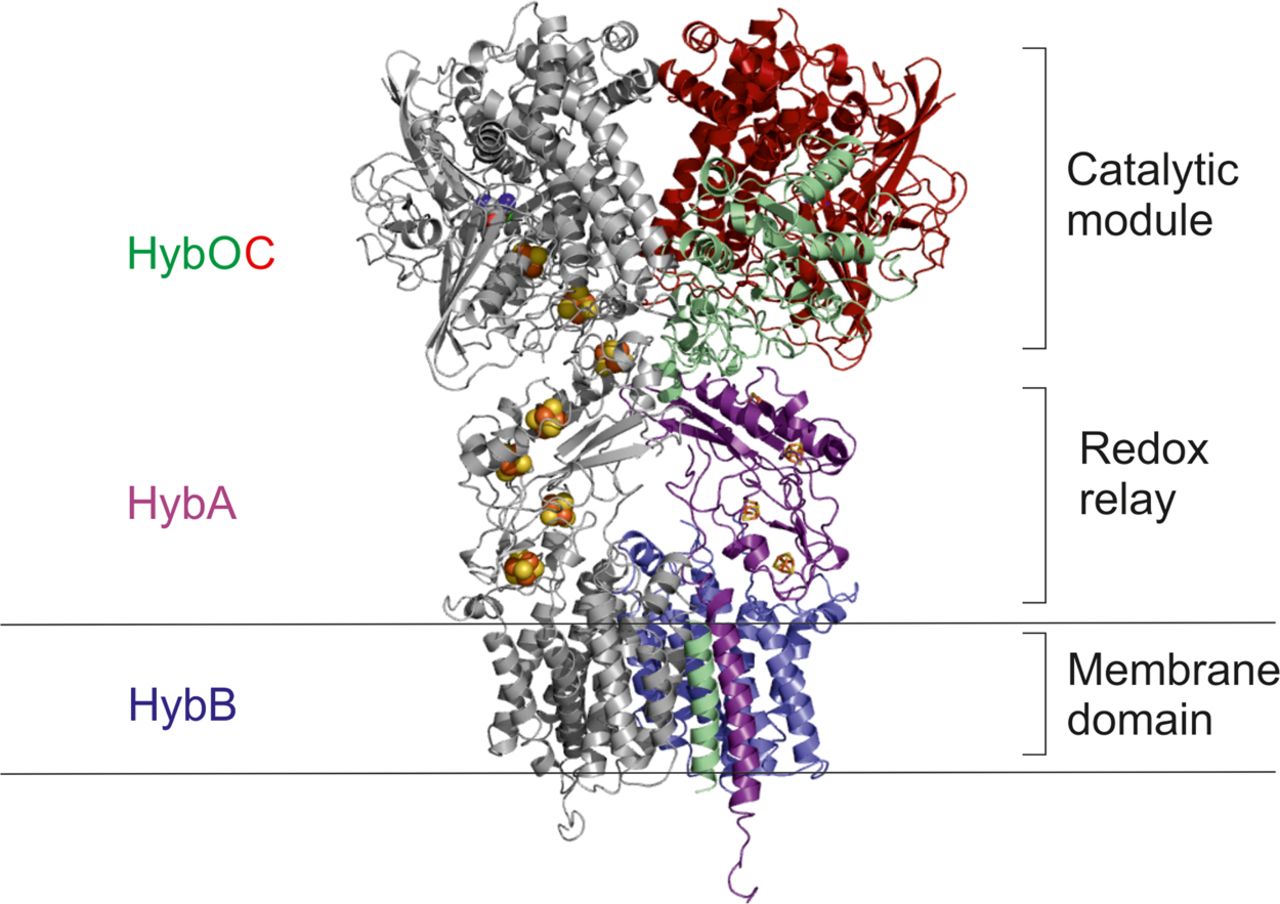
The structure of hydrogenase-2 from Escherichia coli: implications for H2-driven proton pumping
Stephen E. Beaton, Rhiannon M. Evans, Alexander J. Finney, Ciaran M. Lamont, Fraser A. Armstrong, Frank Sargent, Stephen B. Carr
Biochemical Journal
[Link]
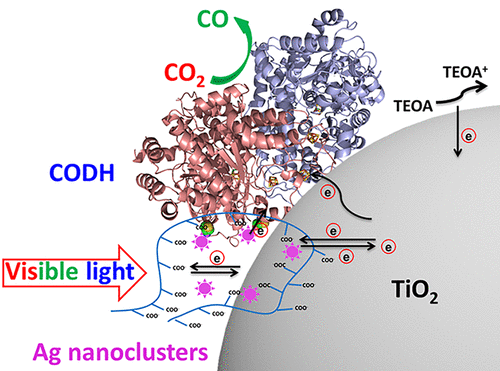
Fast and Selective Photoreduction of CO2 to CO Catalyzed by a Complex of Carbon Monoxide Dehydrogenase, TiO2, and Ag Nanoclusters
Liyun Zhang, Mehmet Can, Stephen W. Ragsdale, and Fraser A. Armstrong
ACS Catal.
[Link]
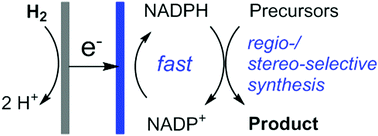
A hydrogen fuel cell for rapid, enzyme-catalysed organic synthesis with continuous monitoring
L. Wan, C. F. Megarity, B. Siritanaratkul and F. A. Armstrong
Chem. Commun.
[Link]
Protein Film Electrochemistry of Iron-Sulfur Enzymes
Fraser A.Armstrong, Rhiannon M.Evans, Clare F. Megarity
Methods in Enzymology
[Link]
The radical-SAM enzyme Viperin catalyzes reductive addition of a 5'-deoxyadenosyl radical to UDP-glucose in vitro
K. H. Ebrahimi, S. B. Carr, J. McCullagh, J. Wickens, N. H. Rees, J. Cantley and F. A. Armstrong
FEBS Lett.
[Link]

Frequency and potential dependence of reversible electrocatalytic hydrogen interconversion by [FeFe]-hydrogenases
Kavita Pandey, Shams T. A. Islam, Thomas Happe and Fraser A. Armstrong
PNAS
[Link]
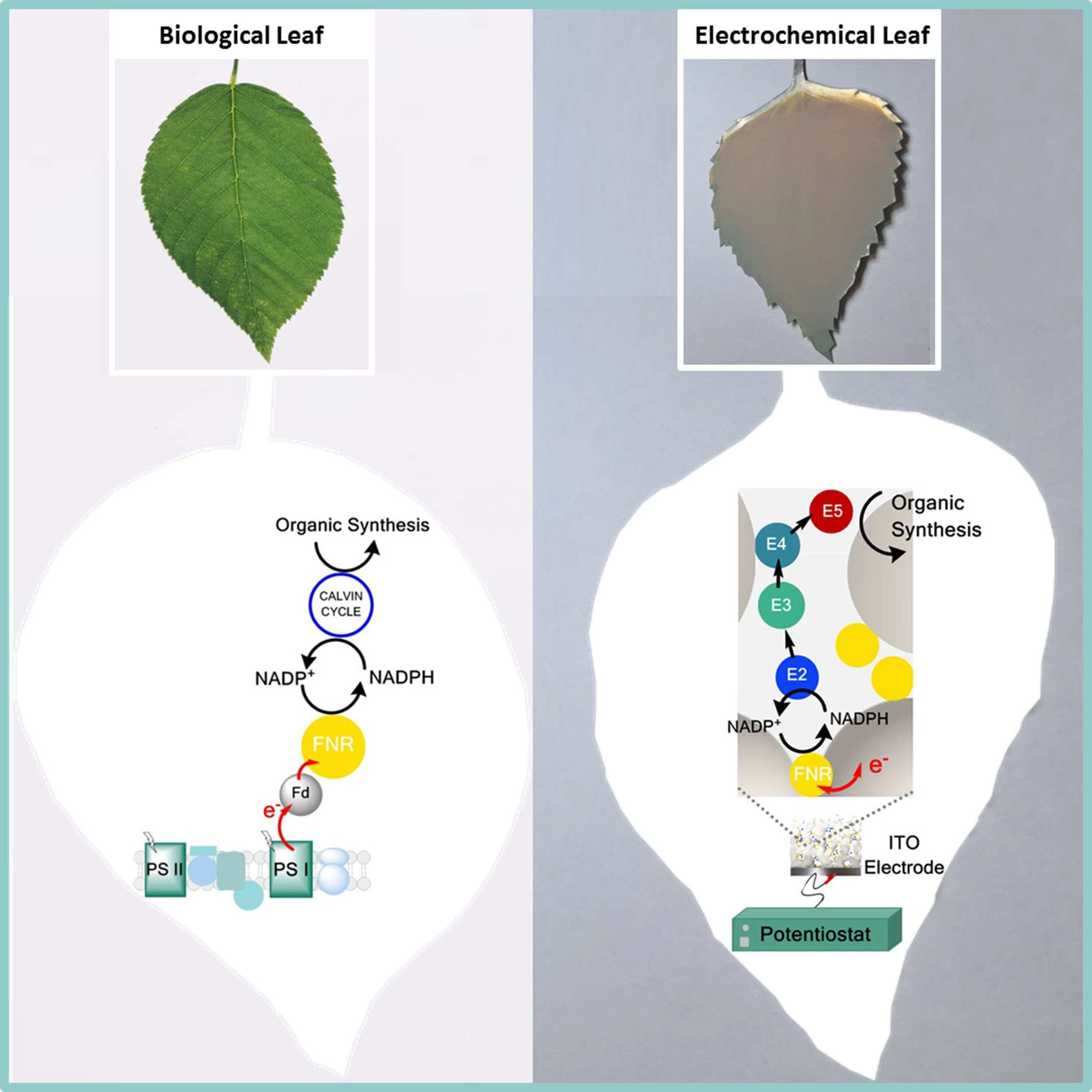
Transfer of photosynthetic NADP+/NADPH recycling activity to a porous metal oxide for highly specific, electrochemically-driven organic synthesis
Bhavin Siritanaratkul, Clare F. Megarity, Thomas G. Roberts, Thomas O. M. Samuels, Martin Winkler, Jamie H. Warner, Thomas Happe and Fraser A. Armstrong
Chem. Sci.
[Link]

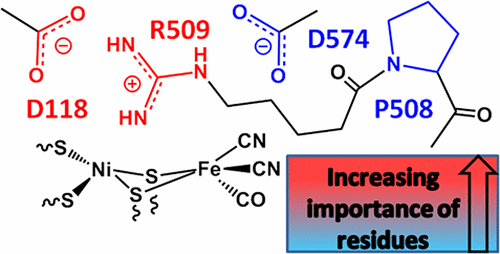
Importance of the active site “canopy” residues in an O2-tolerant [NiFe]-hydrogenase
Emily J. Brooke, Rhiannon M. Evans, Shams T. A. Islam, Gerri M. Roberts, Sara A. M. Wehlin, Stephen B. Carr, Simon E. V. Phillips, and Fraser A. Armstrong
Biochemistry
[Link]
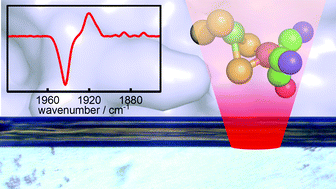
Generating single metalloprotein crystals in well-defined redox states: electrochemical control combined with infrared imaging of a NiFe hydrogenase crystal
P. A. Ash, S. B. Carr, H. A. Reeve, A. Skorupskaite, J. S. Rowbotham, R. Shutt, M. D. Frogley, R. M. Evans, G. Cinque, F. A. Armstrong and K. A. Vincent
Chem. Commun.
[Link]
Electrochemical Investigations of the Mechanism of Assembly of the Active-Site H-Cluster of [FeFe]-Hydrogenases
Clare F. Megarity, Julian Esselborn, Suzannah V. Hexter, Florian Wittkamp, Ulf-Peter Apfel, Thomas Happe, and Fraser A. Armstrong
J. Am. Chem. Soc.
[Link]
Hydrogen activation by [NiFe]-hydrogenases
Stephen B. Carr, Rhiannon M. Evans, Emily J. Brooke, Sara A.M. Wehlin, Elena Nomerotskaia, Frank Sargent, Fraser A. Armstrong, Simon E.V. Phillips
Biochemical Society Transactions
[Link]
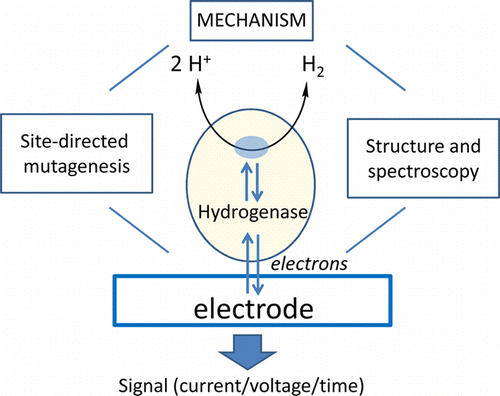
Guiding Principles of Hydrogenase Catalysis Instigated and Clarified by Protein Film Electrochemistry
Fraser A. Armstrong, Rhiannon M. Evans, Suzannah V. Hexter, Bonnie J. Murphy, Maxie M. Roessler, and Philip Wulff
Acc. Chem. Res.
[Link]
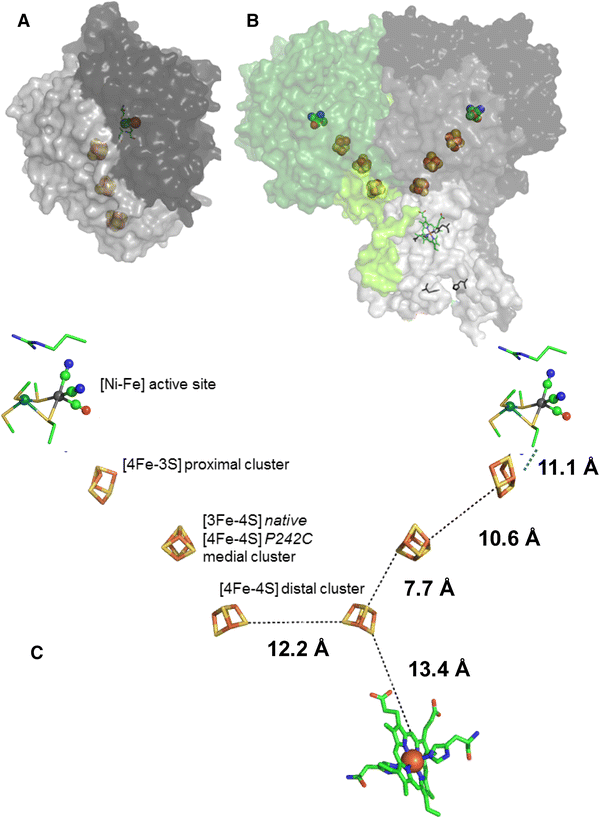
How the oxygen tolerance of a [NiFe]-hydrogenase depends on quaternary structure
Philip Wulff, Claudia Thomas, Frank Sargent, Fraser A. Armstrong
J. Biol. Inorg. Chem.
[Link]
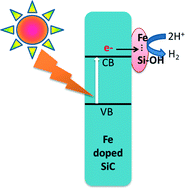
Catalysis of solar hydrogen production by iron atoms on the surface of Fe-doped silicon carbide
Zhijiang Wang and Fraser A. Armstrong
Catal. Sci. Technol.
[Link]
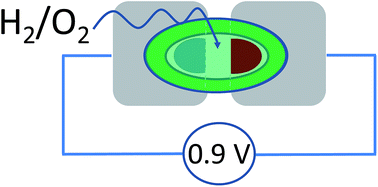
Electrocatalysis by H2-O2membrane-free fuel cell enzymes in aqueous microenvironments confined by an ionic liquid
Yiduo Wang, Thomas F. Esterle and Fraser A. Armstrong
RSC Adv.
[Link]
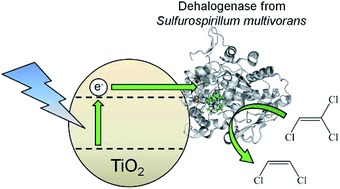
Selective, light-driven enzymatic dehalogenations of organic compounds
Bhavin Siritanaratkul, Shams T. A. Islam, Torsten Schubert, Cindy Kunze, Tobias Goris, Gabriele Diekert and Fraser A. Armstrong
RSC Adv.
[Link]
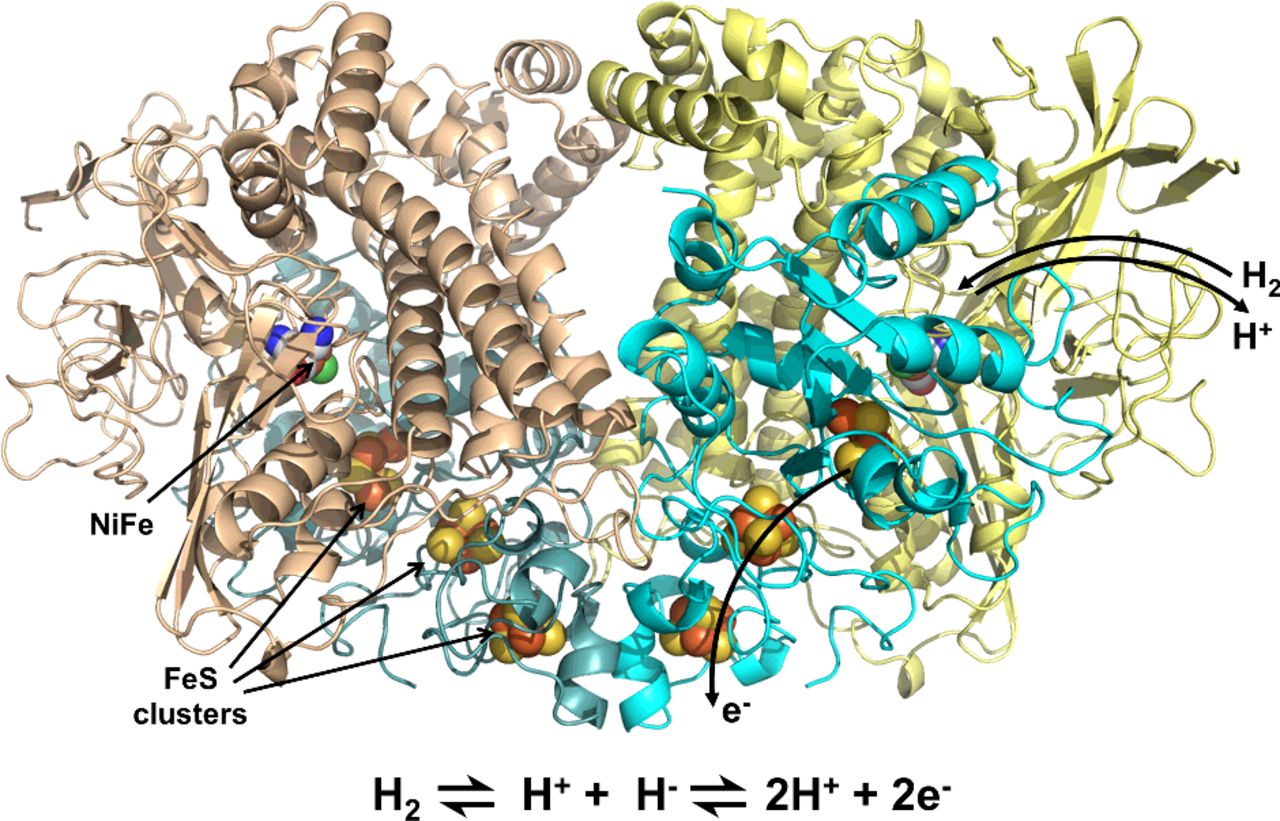
Mechanism of hydrogen activation by [NiFe] hydrogenases
Rhiannon M Evans, Emily J Brooke, Sara A M Wehlin, Elena Nomerotskaia, Frank Sargent, Stephen B Carr, Simon E V Phillips and Fraser A Armstrong
Nature Chem. Biol.
[Link]